drug dilution guidelines
Each drug is limited to one or two recommended concentrations that will serve the needs of most patients in the care settings where the drug is most commonly used. Dilution should never be done with a manufactured prefilled saline flush syringe.
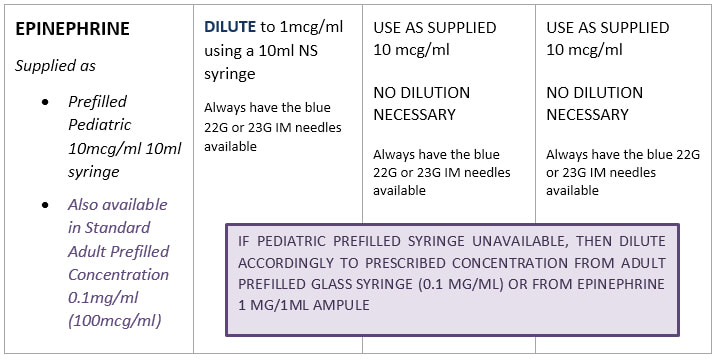
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
However this procedure may also be considered compounding.
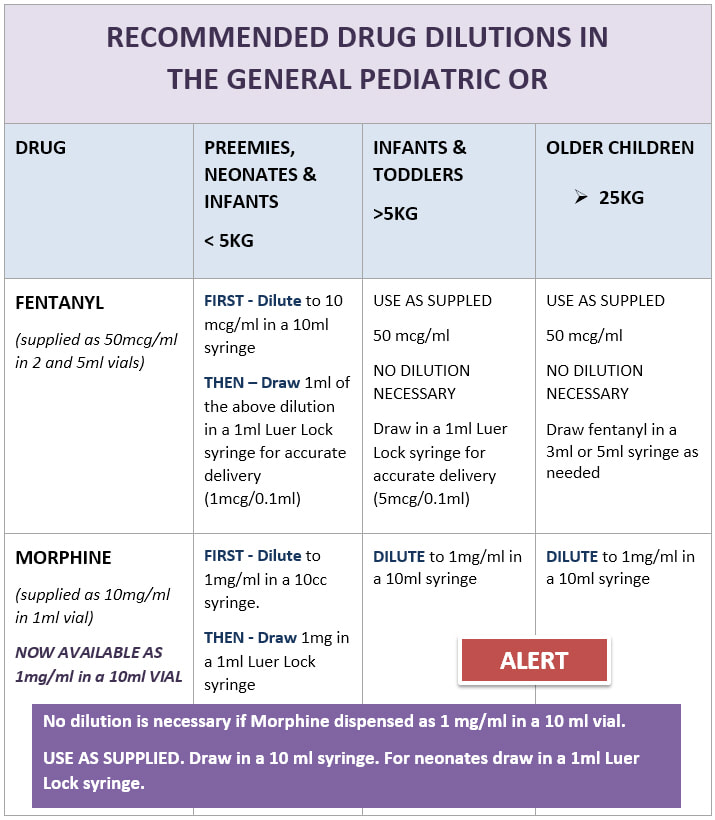
. X Dilute to 2 InfusionMAX concentration of 2 mgmL over 1 hr Dosing. However this procedure is considered compounding. Vanessa Henke Joyce Chang and Linda Liu Drug and Standard Concentration for Infusion How supplied Dilution Instructions Sample Vial Amiodarone 18mg mL Use Losorb non-latex non-DEHP tubing with 02micron filter 150 mg 3 mL 50 mg mL Add 450 mg 9 mL of concentrated amiodarone to 250 mL normal saline.
UMB Animal Care and Use Program. 5-10 mgkgday as q 24 h MAX 500 mg Single dose regimen. 30mgkg X 1 MAX 1500mg For specific indications please consult pedi reference for recommendations.
Expiration Guidelines on Diluted Drugs and Fluids 1. Dilute 50mg in 500ml NS 10ml 1mghr Start infusion at 15mlhr 15mghr Increase 15mlhr every 15min Aim decrease. 052421 Page 1 of 2 Animal Care and Use Program Drug Dilution.
Up to 24 cash back Dilute the 1 g vial with 95 mL of SWI to obtain a final concentration of 100 mgmL. The information provided in this guideline mainly extracted from the package insert for the. Dilution of IV push medications should be done only when either required by the manufacturer based on peer-reviewed evidence or by institutional guidelines.
January 20 2021 _____ Drug Dilution and Storage Guidelines Most of the drugs used for laboratory animals ie mice rats need to be diluted for accurate dosing. The 8020 rule the principle that roughly 80 of the effects come from 20 of the causes will be applicable. Establish Dilution Guidelines for Injectable Drugs topromote safe medication use.
Therefore correct dilution and administration of injectable medications are crucial to ensure patients safety. Guidelines for the safe practice of total intravenous anaesthesia TIVA. UMMC Pediatrics Dilution Protocol for Antimicrobials Antifungals and Antivirals.
Take 5mL 500mg from the reconstituted solution and mix final concentration of 50 mgmL or 500 mg10 mL. Compounding is defined as. No respondents described a dilution process that would result in a specific concentration eg add 4 mL of diluent to 1 mL of drug to equal xx mg5 mL.
Hydralazine For preeclampsia eclampsia Method 1. Dilute with 495 ml of Normal Saline 50 mls dilution Rate. Aztreonam X Azactam Dilute to 20 mgmL IVP.
Monitor for pain at infusion site LFTs WBC and infection. Intravenous admixtures preparation and infusion guidelines. All drugs requiring dilution will be diluted and maintained using sterile technique.
Moreover errors in intravenously administered medications may have serious consequences and incident can happen during preparationdispensing and administration of these drugs. Each monograph contains stability data. As suggested by the variability of dilution methods and volumes less than half of respondents 43 reported organizational policies or guidelines on dilution.
Sterile needles and syringes should be used to transfer drugs and diluents into sterile containers b. In terms of dilution 1 is the same as a 1100 solution dilution. This guideline was prepared to facilitate the healthcare staffs in preparing the dilutions of drugs which are available in the Ministry.
To enable screen reader support press CtrlAltZ To learn about keyboard shortcuts press Ctrlslash. Institutions should provide the appropriate diluent along with the drug to ensure safety. Dilute 50mg in 50cc NS 1mg 1ml Start 5mlhr Increase 1mlhr every 15-20min Max infusion rate 10mlhr Method 2.
No other dilution required Dose of 99 mg and less. Turn on screen reader support. 1ml hour 1 IU hour Ref.
Clinical Practice Guidelines on Management of Type 2 DM KKM 2015 Hospital Sultanah Aminah Guidelines for Drug Reconstitution 2013 Commence Fixed Rate IV Insulin Infusion 005 to 01 Ukghr based on estimate weight. No respondents described a dilution process that would result in a specific concentration eg add 4 mL of diluent to 1 mL of drug to equal xx mg5 mL. Antimicrobial Antifungal and Antiviral Drugs in Pediatrics University Malaya Medical Center - Google Docs.
Concentrations are expressed as amount of drugmL. Dilution reconstitution or the safe rate of administration of IV push medications Risks Associated with Drug Labeling Packaging and Nomenclature IV medications that are prepared in empty sterile syringes but left unlabeled IV push medications that are prepared diluted reconstituted in commercially available syringes of 09 sodium. As suggested by the variability of dilution methods and volumes less than half of respondents 43 reported organizational policies or guidelines on dilution.
Expiration date which is thirty 30 days after the dilution date provided this does not pass either the expiration date of the drug listed on the original container or the sterile diluent see 5 below. So if there is 1g per 100mls there is also 1000mg per 100mls. All diluted drugs must be labeled with the following information.
This reference contains standard dilutions including IV admixture drug concentration infusion volumes and infusion rates. Bagshaw O et al. Prepare a solution at 50 mgmL with 5 mL of SWI to obtain a 10 mL solution.
Drug Dilution Storage IACUC Approval Date. There are a few types of medication errors related to injectable. Dilution Guideline for Injectable Drugs serves as a general reference for the healthcare professionals in the Ministry of Health on how to prepare dilution of certain injectable drugs before administering to the patient.
The resident who was unfamiliar with the process of drug dilution assumed that this should be diluted into 2 mL to mimic the premade syringes and mixed 1 mg of remifentanil powder into a syringe of 2 mL saline at a concentration of 500 mcgmL. 14 April 2021 - 319pm. If we divide this down there will be 10mg in 1ml.
5ml of lidocaine will have 5 x 10mg 50mg in a 5ml vial. Drug Dilution and Storage Guidelines DATE. Dose of 100 mg and more.
A 1 solution literally means there is 1gram per 100mls. Name of person who made the dilution. Version 1 11321 Contributors.

Photo 46 Medication Dilution Chart Posted At The Nursing Station For Download Scientific Diagram

Drug Dilutions Used For Intradermal Skin Testing Download Table
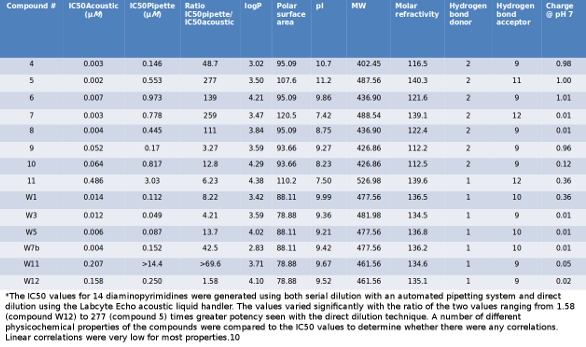
Direct Improvement With Direct Dilution American Laboratory
Wall Chart Drugs Given By Iv Push Or Rapid Administration In Adults Institute For Safe Medication Practices
Intravenous Iv Preparation And Infusion Guidelines Globalrph
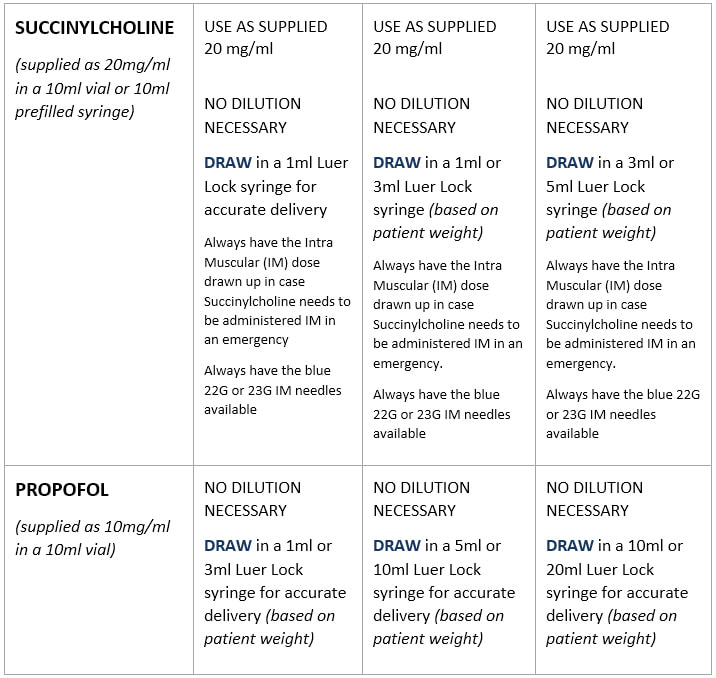
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
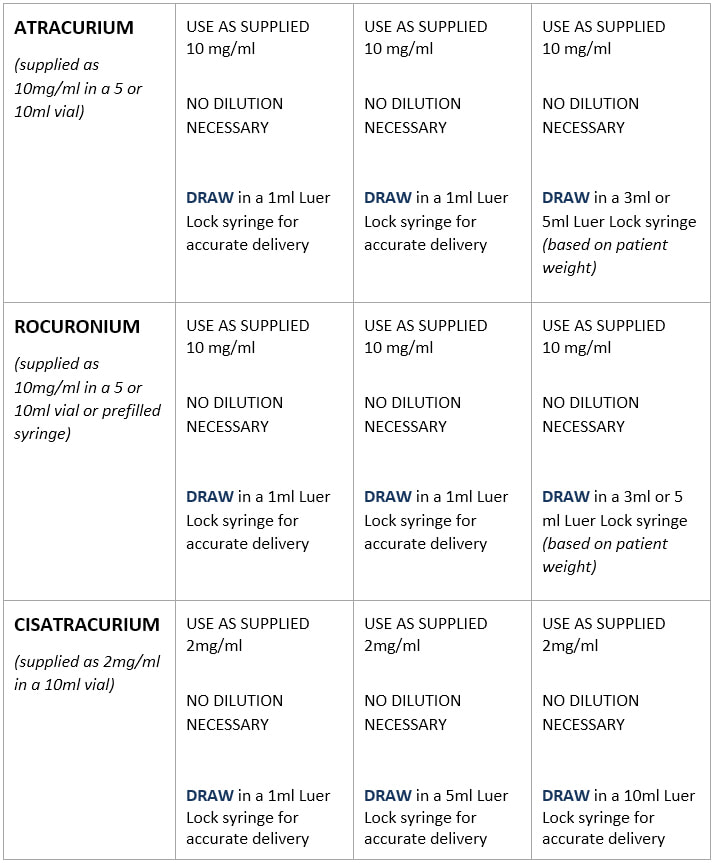
Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices

Standard Drug Dilutions In The Pediatric Or Pediatric Anesthesia Digital Handbook

Intravenous Iv Preparation And Infusion Guidelines Globalrph
Some Iv Medications Are Diluted Unnecessarily In Patient Care Areas Creating Undue Risk Institute For Safe Medication Practices

Photo 46 Medication Dilution Chart Posted At The Nursing Station For Download Scientific Diagram
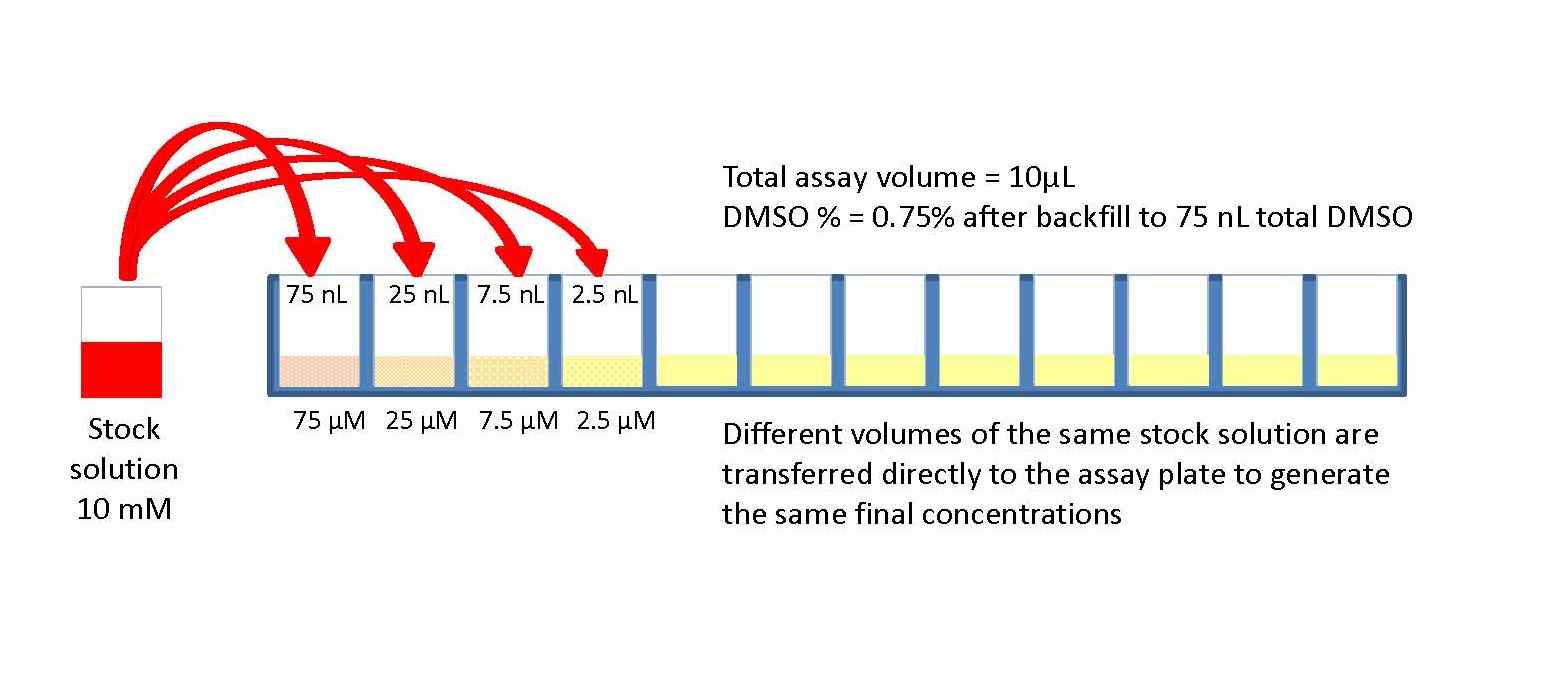
Direct Improvement With Direct Dilution American Laboratory







Comments
Post a Comment